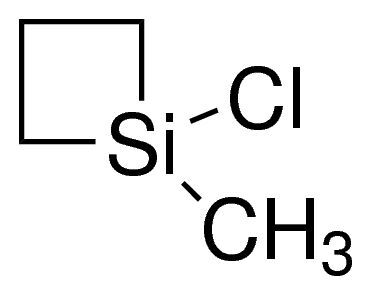
1-Chloro-1-methylsilacyclobutane / 5 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 3 °C - closed cup |
| Flash Point (F) | 37.4 °F - closed cup |
| Density | 0.985 g/mL at 25 °C (lit.) |
| Boiling Point | 103 °C (lit.) |
Product Description
"1-CHLORO-1-METHYLSILACYCLOBUTANE is a chemical compound used in organic synthesis for the preparation of various silicon-containing molecules. It serves as a valuable reagent in the creation of silicon-based intermediates and compounds."
Application:
"Applied in organic synthesis, 1-CHLORO-1-METHYLSILACYCLOBUTANE functions as a crucial reagent for the generation of silicon-containing intermediates, playing a significant role in the development of diverse chemical compounds."
Articles:
- Raman and infrared spectra, conformational stability, normal coordinate analysis, ab initio calculations and vibrational assignment of 1-chloro-1-methylsilacyclobutane
Publication Date: 10 May 1999
Todor K. Gounev, Gamil A. Guirgis, Tarek A. Mohamed, Pengqian Zhen, James R. Durig
https://doi.org/10.1002/(SICI)1097-4555(199905)30:5<399::AID-JRS382>3.0.CO;2-F
- Infrared, and Raman spectra, conformational stability, normal coordinate analysis, ab initio calculations, and vibrational assignment of 1-chlorosilacyclobutane
Publication Date: Available online 6 June 2000
J.R. Durig, T.K. Gounev, P. Zhen, G.A. Guirgis
https://doi.org/10.1016/S0166-1280(00)00381-X
- An improved cyclization procedure for 3-chloropropylchlorosilanes: Efficient syntheses of silacyclobutanes
Publication Date: Available online 4 June 2001
R. Damrauer, R.A. Davis, M.T. Burke, R.A. Karn, G.T. Goodman