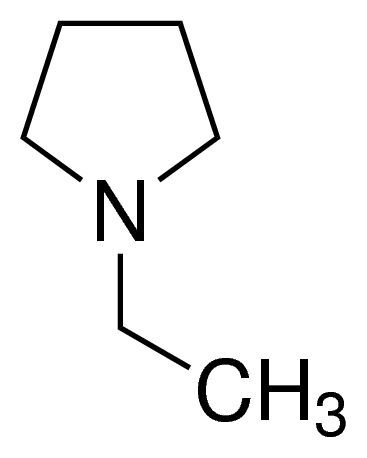
1-Ethylpyrrolidine / 5 ML
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | -22 °C |
| Flash Point (F) | -7.6 °F |
Product Description
1-Ethylpyrrolidine is a heterocyclic organic compound consisting of a pyrrolidine ring with an ethyl group attached to the nitrogen atom. It is a colorless liquid used in chemical research and synthesis.
Application
1-Ethylpyrrolidine is utilized as an intermediate in the synthesis of pharmaceuticals and agrochemicals. It serves as a building block in various organic reactions, aiding in the development of complex molecules.
Articles:
- How do prolonged anchorage-free lifetimes strengthen non-small-cell lung cancer cells to evade anoikis? – A link with altered cellular metabolomics
Publication Date: 05 August 2023
Rungroch Sungthong, Hnin Ei Ei Khine, Somruethai Sumkhemthong, Pithi Chanvorachote, Rossarin Tansawat & Chatchai Chaotham
https://doi.org/10.1186/s40659-023-00456-z
- Iodine-catalyzed sulfonylation of sulfonyl hydrazides with tert-amines: a green and efficient protocol for the synthesis of sulfonamides
Publication Date: 2nd October 2019
Jinyang Chen, Xiaoran Han, Lan Mei, Jinchuan Liu, Kui Du, Tuanwu Cao and Qiang Li
https://doi.org/10.1039/C9RA07361B
- DEVELOPMENT OF AN EFFICIENT HPLC METHOD WITH PRE-COLUMN DERIVATIZATION FOR DETERMINATION OF ENANTIOMERIC PURITY OF 2-(AMINOMETHYL)-1-ETHYLPYRROLIDINE
Publication Date: mar. 2015
DAO-CAI WANG, TIAN YAO, GUO-FEI QIAN, LING-XIANG PU, SHUN YAO, HANG SONG