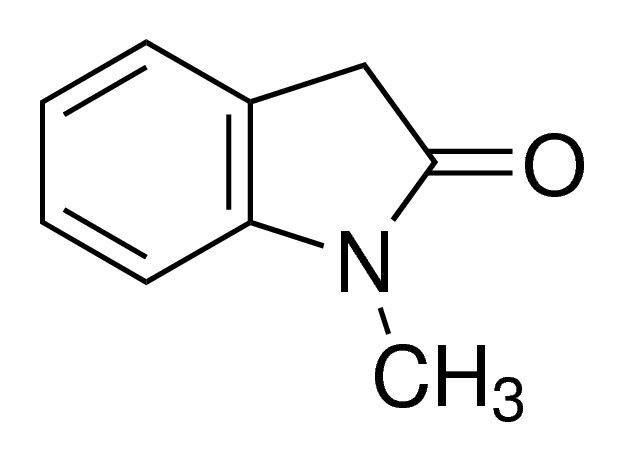
1-Methyl-2-oxindole / 5 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 85-88 °C (lit.) |
Product Description
1-Methyl-2-oxindole is a chemical compound commonly used as an intermediate in the synthesis of pharmaceuticals and agrochemicals. It possesses unique structural properties that make it valuable in organic synthesis processes.
Application:
1-Methyl-2-oxindole is employed as a key building block in the production of various pharmaceuticals and agrochemicals, contributing to the synthesis of diverse compounds in these industries.
Articles:
- Aromatic and heteroaromatic annelation studies on 3-[bis(methylthio)methylene]-1-methyloxindole: synthesis of carbazoles and an efficient route to pyrido[2,3-b]indoles
Publication Date: Available online 15 January 2001
J.R Suresh, U.K Syam Kumar, H Ila, H Junjappa
https://doi.org/10.1016/S0040-4020(00)01054-1
- Quercetin and 1-methyl-2-oxindole mimic root signaling that promotes spore germination and mycelial growth of Gigaspora margarita
Publication Date: 22 February 2022
Alberto Campos-López, Jaime A. Uribe-López, Verna Cázares-Ordoñez, Roberto Garibay-Orijel, Norma A. Valdez-Cruz & Mauricio A. Trujillo-Roldán
https://doi.org/10.1007/s00572-022-01074-5
- An efficient one-pot synthesis of spiro dihydrofuran oxindole and spiro 2-hydroxytetrahydrofuran oxindole derivatives via (3+2) oxidative cycloaddition mediated by CAN
Publication Date: Available online 15 February 2007
G. Savitha, S.K. Niveditha, D. Muralidharan, P.T. Perumal