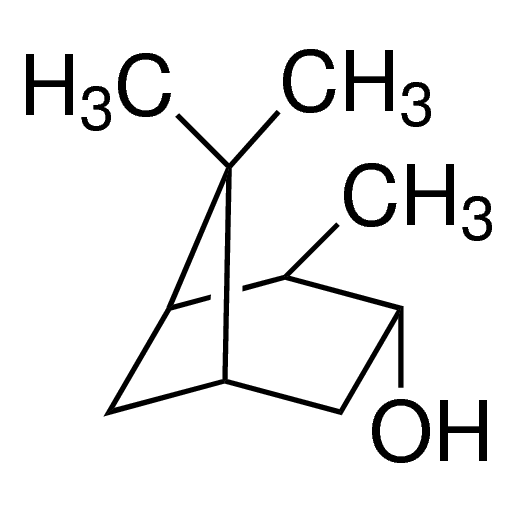
(1R,2R,3R,5S)-(−)-Isopinocampheol / 25 G
Properties
| Flash Point (C) | 93.4 °C - closed cup |
| Boiling Point | 219 °C (lit.) |
| Melting Point | 51-53 °C (lit.) |
Product Description
(1R,2R,3R,5S)-(−)-Isopinocampheol is a chiral compound with a bicyclic structure. It is commonly used as a chiral auxiliary in asymmetric synthesis and as a building block in organic chemistry.
Application:
(1R,2R,3R,5S)-(−)-Isopinocampheol is primarily employed as a chiral auxiliary in asymmetric synthesis to control the stereochemistry of reactions. It also serves as a precursor for the synthesis of various complex molecules in organic chemistry.
Articles:
- Reduction of 2, 3-epoxypinane and 3, 4-epoxyc arane
Publication Date: June 1959
Z. G. Isaeva & B. A. Arbuzov
https://doi.org/10.1007/BF00916669
- Proton Transfer Reaction - Mass Spectrometry Determination of the Emission Rate of Flavor Ingredients in Toothpastes
Publication Date: February 2013
Ge Zhang, Sheng Nan Gao, Wen Ding Wei, Qiu Jian Xu
https://doi.org/10.4028/www.scientific.net/AMM.299.229
- Zur Kenntnis des Rosenöls. 2. Mitteilung. Die Konstitution des Oxyds C10H18O aus bulgarischem Rosenöl
Publication Date: 1961
C. F. Seidel, Dorothee Felix, A. Eschenmoser, K. Biemann, E. Palluy, M. Stoll