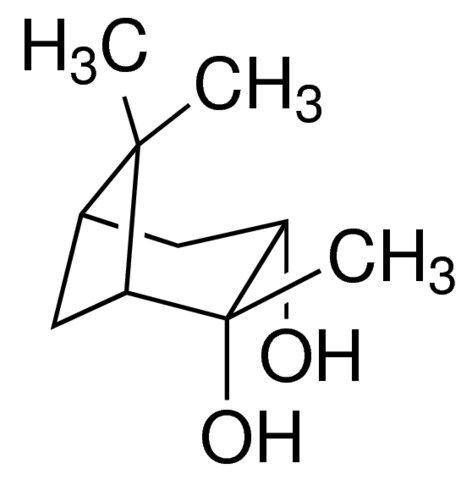
(1R,2R,3S,5R)-(−)-Pinanediol / 5 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | 110 °C - closed cup |
| Flash Point (F) | 230.0 °F - closed cup |
| Boiling Point | 101-102 °C/1 mmHg (lit.) |
| Melting Point | 57-59 °C (lit.) |
Product Description
(1R,2R,3S,5R)-(−)-Pinanediol is a chiral diol derived from pinene. It is used as a chiral auxiliary in asymmetric synthesis and various organic reactions.
Application:
(1R,2R,3S,5R)-(−)-Pinanediol is employed in the synthesis of enantiomerically pure compounds, serving as a chiral auxiliary to induce stereochemistry in target molecules.
Articles:
- Organoboranes: LI. Convenient procedures for the recovery of pinanediol in asymmetric synthesis via one-carbon homologation of boronic esters
Publication Date: Available online 4 May 2001
Herbert C. Brown, Milind V. Rangaishenvi
https://doi.org/10.1016/0022-328X(88)87067-0
- Two efficient methods for the cleavage of pinanediol boronate esters yielding the free boronic acids
Publication Date: Available online 9 March 2001
Simon J. Coutts, Julian Adams, Dale Krolikowski, Roger J. Snow
https://doi.org/10.1016/S0040-4039(00)77040-7
- Asymmetric synthesis of alkylarylcarbinols via reaction of a chiral pinanediol alkylboronic ester with arylmethyl chlorides
Publication Date: Available online 14 April 1998
George W. Kabalka, Nan-Sheng Li, Su Yu