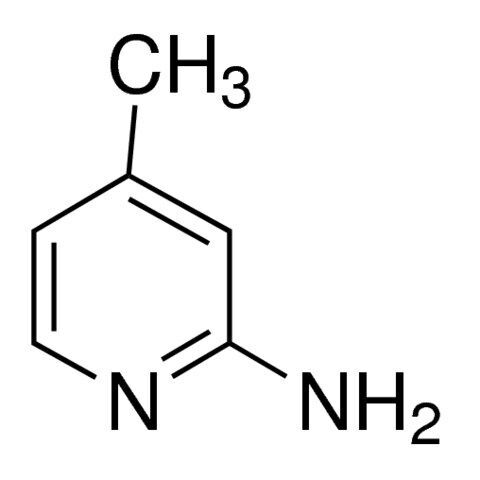
2-Amino-4-methylpyridine / 100 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Danger |
| Flash Point (C) | 118 °C - closed cup |
| Flash Point (F) | 244.4 °F - closed cup |
| Boiling Point | 230 °C (lit.) |
| Melting Point | 96-99 °C (lit.) |
Product Description
2-AMINO-4-METHYLPYRIDINE is a chemical compound often used as a building block in the synthesis of pharmaceuticals, agrochemicals, and organic compounds due to its functional groups and reactivity.
Application:
2-AMINO-4-METHYLPYRIDINE is commonly employed in the pharmaceutical and agrochemical industries as a key intermediate in the production of various drugs and crop protection chemicals.
Articles:
- 2-Amino-4-methylpyridine as a potent inhibitor of inducible NO synthase activity in vitro and in vivo
Publication Date: November 1996
W. Stephen Faraci, Arthur A. Nagel, Kimberly A. Verdries, Lawrence A. Vincent, Hong Xu, Lois E. Nichols, Jeffrey M. Labasi, Eben D. Salter, E. Roy Pettipher
https://doi.org/10.1111/j.1476-5381.1996.tb16010.x
- Inhibition of Nitric Oxide Synthase Aggravates Cisplatin-Induced Nephrotoxicity: Effect of 2-Amino-4-Methylpyridine
Publication Date: APRIL 10 2003
Sherif Y. Saad; Tawfeeg A.O. Najjar; Mohammed H. Daba; Ammar C. Al-Rikabi
https://doi.org/10.1159/000069714
- Hydrogen Bonded Supramolecular Association in Organic Acid–Base Salts: Crystal Structures of Four Proton-Transfer Complexes Assembled from 2-Amino-4-methylpyridine with 2-Chloro, 4-Chloro, 3-Methyl and 4-Methylbenzoic Acid
Publication Date: 07 October 2014
Nuridayanti Che Khalib, Kaliyaperumal Thanigaimani, Suhana Arshad & Ibrahim Abdul Razak