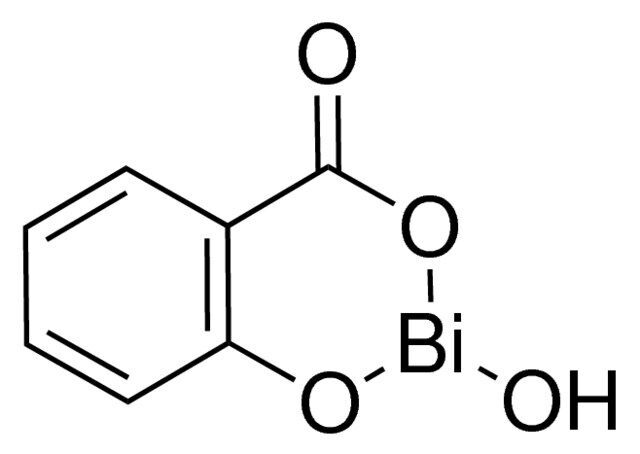
Bismuth(III) subsalicylate / 500 G
Properties
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | >350 °C (lit.) |
Product Description
Bismuth subsalicylate is an over-the-counter medication commonly used to treat diarrhea, indigestion, and heartburn. It works by reducing the secretion of fluids and inflammation in the digestive system.
Application:
Bismuth subsalicylate is applied orally to alleviate symptoms associated with gastrointestinal issues such as diarrhea, upset stomach, and nausea. It helps to relieve discomfort by soothing the lining of the digestive tract and reducing diarrhea frequency.
Articles:
- Metabolism of Bismuth Subsalicylate and Intracellular Accumulation of Bismuth by Fusarium sp. Strain BI
Publication Date: 1 February 2005
Anthony G. Dodge, Lawrence P. Wackett
https://doi.org/10.1128/AEM.71.2.876-882.2005
- A Controlled Trial of Bismuth Subsalicylate in Infants with Acute Watery Diarrheal Disease
Publication Date: June 10, 1993
Dante Figueroa-Quintanilla, Eduardo Salazar-Lindo, R. Bradley Sack, Raul Leon-Barua, Silvana Sarabia-Arce, Miguel Campos-Sanchez, and Eduardo Eyzaguirre-Maccan
https://doi.org/10.1056/NEJM199306103282301
- Effect of Bismuth Subsalicylate on Gastrointestinal Tolerability in Healthy Volunteers Receiving Oral Delayed-release Dimethyl Fumarate: PREVENT, a Randomized, Multicenter, Double-blind, Placebo-controlled Study
Publication Date: Available online 15 November 2018
Irene Koulinska, Katherine Riester, Spyros Chalkias, Michael R. Edwards