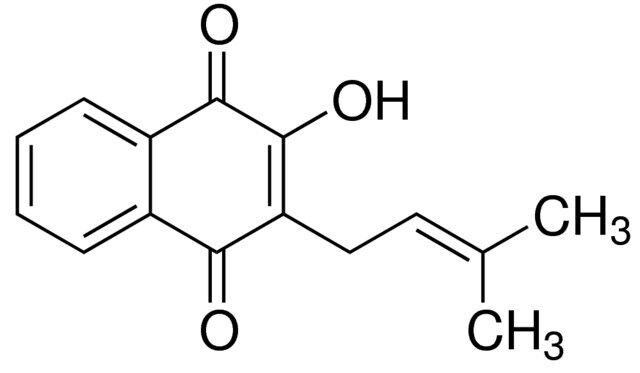
Lapachol / 20 MG
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Signal Word | Warning |
| Flash Point (C) | Not available |
| Flash Point (F) | Not available |
| Melting Point | 141-143 °C (lit.) |
Product Description
Lapachol is a natural compound found in the bark of certain trees, including the lapacho tree. It is known for its potential medicinal properties and has been studied for its anticancer and antimicrobial effects.
Application:
Lapachol is investigated for its potential therapeutic benefits, particularly in cancer treatment and as an antimicrobial agent. It may be used in natural medicine formulations and pharmaceutical research aimed at developing new treatments for cancer and infectious diseases.
Articles:
- The plant-derived naphthoquinone lapachol causes an oxidative stress response in Staphylococcus aureus
Publication Date: Available online 24 July 2020
Nico Linzner, Verena Nadin Fritsch, Tobias Busche, Quach Ngoc Tung, Vu Van Loi, Jörg Bernhardt, Jörn Kalinowski, Haike Antelmann
https://doi.org/10.1016/j.freeradbiomed.2020.07.025
- Lapachol inhibits glycolysis in cancer cells by targeting pyruvate kinase M2
Publication Date: February 2, 2018
Mani Shankar Babu, Sailendra Mahanta, Alexander J. Lakhter, Takashi Hato, Subhankar Paul, Samisubbu R. Naidu
https://doi.org/10.1371/journal.pone.0191419
- The antimicrobial activity of lapachol and its thiosemicarbazone and semicarbazone derivatives
Publication Date: May 2013
Marina Azevêdo Souza, Susana Johann, Luciana Alves Rodrigues dos Santos Lima, Fernanda Fraga Campos, Isolda Castro Mendes, Heloisa Beraldo, Elaine Maria de Souza-Fagundes, Patrícia Silva Cisalpino, Carlos Augusto Rosa, Tânia Maria de Almeida Alves, Nívea Pereira de Sá, Carlos Leomar Zani