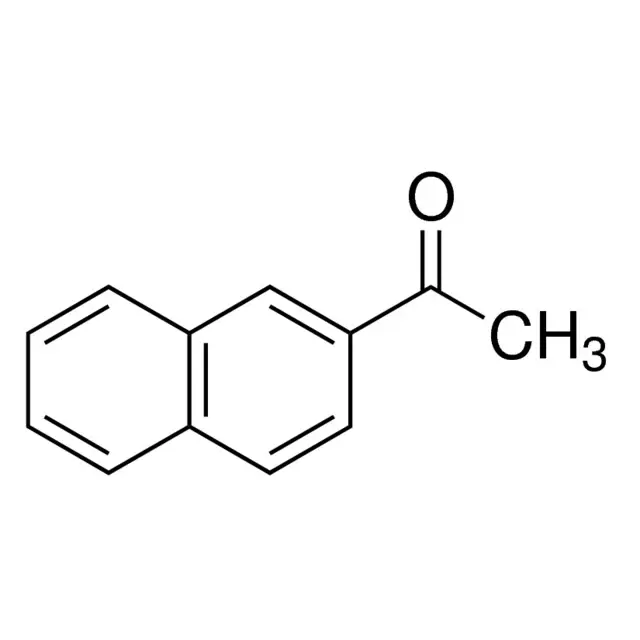
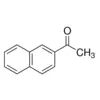
Methyl β-naphthyl ketone / 100 G
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Properties
| Flash Point (C) | 168 °C - DIN 51758 |
| Flash Point (F) | 334.4 °F - DIN 51758 |
| Boiling Point | 300-301 °C (lit.) |
| Melting Point | 52-56 °C (lit.) |
Product Description
2-Acetonaphthone is a chemical compound commonly used in various industrial processes. It serves as a key intermediate in the synthesis of pharmaceuticals, dyes, and other organic compounds.
Application:
Widely employed in the pharmaceutical and chemical industries, 2-Acetonaphthone is primarily utilized as a crucial building block for the production of diverse chemical substances, including pharmaceuticals and dyes.
Articles:
- Schiff bases of 1′-hydroxy-2′-acetonaphthone containing chalcogen functionalities and their complexes with and (p-cymene)Ru(II), Pd(II), Pt(II) and Hg(II): Synthesis, structures and applications in C–C coupling reactions
Publication Date: Available online 29 July 2008
Arun Kumar, Monika Agarwal, Ajai K. Singh
https://doi.org/10.1016/j.jorganchem.2008.07.024
- Ultrafast proton transfer of 1-hydroxy-2-acetonaphthone: Reaction path from resonance Raman and transient absorption studies
Publication Date: 2005
S. Lochbrunner; A. Szeghalmi; K. Stock; M. Schmitt
https://doi.org/10.1063/1.1914764
- NMR study of proton transfer equilibrium in Schiff bases derived from 2-hydroxy-1-naphthaldehyde and 1-hydroxy-2-acetonaphthone. Deuterium isotope effects on 13C and 15N chemical shifts
Publication Date: 05 November 2001
T. Dziembowska, Z. Rozwadowski, A. Filarowski, P. E. Hansen