A-366
Safety Information
Hazard Statements
Precautionary Statements
Pictograms
Product Description
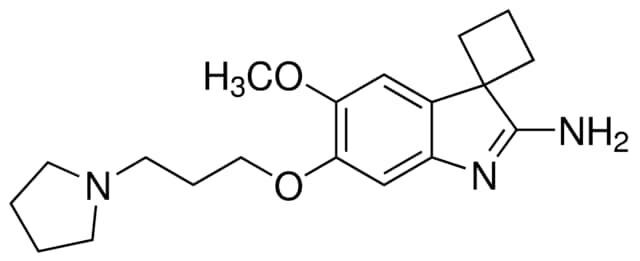
A-366 is a chemical compound known for its role as a selective inhibitor in epigenetic research, specifically targeting a certain enzyme. It is widely used as a tool in biological studies investigating chromatin regulation.
Application:
Primarily employed as a selective inhibitor in epigenetic research, A-366 serves as a valuable tool for studying chromatin regulation, aiding in biological investigations focused on specific enzyme functions.
Articles:
- The Histone Methyltransferase Inhibitor A-366 Uncovers a Role for G9a/GLP in the Epigenetics of Leukemia
Publication Date: July 6, 2015
William N. Pappano ,Jun Guo ,Yupeng He,Debra Ferguson,Sujatha Jagadeeswaran,Donald J. Osterling,Wenqing Gao,Julie K. Spence,Marina Pliushchev,Ramzi F. Sweis,Fritz G. Buchanan,Michael R. Michaelides,Alexander R. Shoemaker,Chris Tse,Gary G. Chiang
https://doi.org/10.1371/journal.pone.0131716
- Ultraviolet radiation-A (366 nm) induced morphological and histological malformations during embryogenesis of Clarias gariepinus (Burchell, 1822)
Publication Date: Available online 20 February 2009
Usama M. Mahmoud, Imman A.A. Mekkawy, Alaa El-Din H. Sayed
https://doi.org/10.1016/j.jphotobiol.2009.02.003
- Abstract 5532: Discovery of A-366, a novel small molecule inhibitor that uncovers an unappreciated role for G9a/GLP in the epigenetics of leukemia
Publication Date: 2014
Jun Guo; Marina Pliushchev; Yupeng He; Debra Ferguson; Sujatha Jagadeeswaran; Andrew Petros; Chaohong Sun; Niru B. Soni; Bailin Shaw; Alla Korepanova; David Maag; Ramzi Sweis; Fritz G. Buchanan; Michael Michaelides; Alex Shoemaker; Chris Tse; Gary G. Chiang; William N. Pappano